Abstract
Background: Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet counts (PCs) caused by a combination of both impaired platelet production and increased peripheral platelet destruction. ITP in children often resolves on its own, but it may become chronic and symptomatic in a proportion of children affected. After failure of first-line therapies (e.g. corticosteroids or immunoglobulin), treatment options for children include immunosuppressants, such as rituximab, and thrombopoietin receptor agonists (TPO-RAs). Avatrombopag (AVA) is an orally administered small molecule TPO-RA. It binds the human c-Mpl at a different site than endogenous TPO, stimulating signal transduction mimicking the biological effects of endogenous TPO in a non-competitive manner. In a phase 3 trial (NCT01438840) in adults with ITP, the primary efficacy endpoint of cumulative number of weeks with PC ≥50×10 9/L during 6 months of treatment in the absence of rescue therapy, was statistically significant favoring AVA over placebo. The most common treatment-emergent adverse events (AEs) in the phase 2 and 3 trials in adults (n=128) were headache, fatigue, and contusion. AVA has no significant hepatotoxicity, is administered with food, and has no restrictions on meal composition. AVA is approved by FDA and EMA for treatment of primary chronic ITP in adult patients with an insufficient response to a previous treatment. For pediatric patients with ITP, there is an unmet need for new treatment options, given the difficult administration requirements and variable, transient response, frequent relapse, and associated toxicities of available treatments.
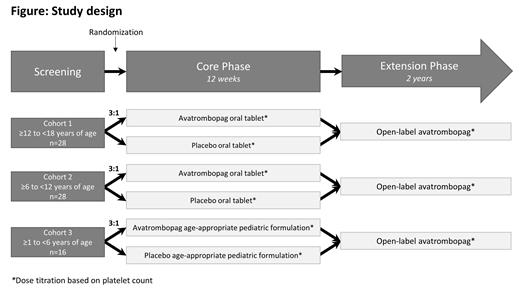
Study Design and Methods: Described here is the rationale and design of a phase 3b multicenter, randomized, double-blind placebo-controlled, parallel-group trial with an open-label extension phase (NCT04516967), evaluating the efficacy and safety of AVA for the treatment of pediatric patients with ITP for ≥6 months with an insufficient response to a previous treatment.
Main inclusion criteria (Core phase) include: Age ≥1 and <18 years; informed consent; primary ITP for ≥6 months duration and an insufficient response to previous treatment; an average of 2 PCs <30×10 9/L, with no single count >35×10 9/L. Main exclusion criteria: secondary ITP; inherited thrombocytopenia; history of arterial or venous thrombosis, myelodysplastic syndrome; or congenital heart abnormalities or arrhythmias.
Subjects will be randomized to blinded therapy of AVA or placebo (3:1 ratio) for 12 weeks, stratified by age cohort and baseline PC (Figure). AVA or placebo will be administered as an oral tablet (20 mg, age cohort 1 and 2) or as an age-appropriate pediatric formulation (10 mg, age cohort 3). Subjects who complete the Core Phase and are eligible may continue to the open label extension phase (2 years).
The primary endpoint is durable platelet response, defined by the proportion of subjects achieving ≥6 out of 8 weekly PCs ≥50×10 9/L during the last 8 weeks of the 12 week treatment period in the Core Phase, in the absence of rescue medication.
Secondary endpoints include proportion of subjects with ≥2 consecutive PCs ≥50×10 9/L during the Core Phase (12 weeks) in the absence of rescue medication; percentage of weeks with PC ≥50×10 9/L, and between ≥50×10 9/L and ≤150×10 9/L, during Core Phase (12 weeks) in the absence of rescue therapy; proportion of subjects with PC ≥50×10 9/L at day 8, proportion of subjects who require rescue medications during Core Phase; incidence and severity of bleeding symptoms; safety and PK/PD parameters.
Statistics: The primary endpoint will be tested using the Cochran-Mantel-Haenszel 2-sided test at α=0.05, adjusting for age cohort and baseline PC (≤15×10 9/L vs >15×10 9/L), or the Fisher's exact test, when data is sparse. In addition, the numbers and percentages of responders in each treatment group, the associated 95% confidence intervals (CI), and the 95% CI for the difference between AVA and placebo will be estimated.
Study Status: This global study is currently enrolling patients and aims to include at least 72 patients in total, at approximately 62 sites.
Grace: Principia: Membership on an entity's Board of Directors or advisory committees; Dova: Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Research Funding; Novartis: Research Funding. Xue: Dova Pharmaceuticals, a Sobi company: Current Employment. Jamieson: Sobi, Inc.: Current Employment.
Avatrombopag is an orally administered thrombopoietin receptor agonist (TPO-RA) that mimics the biologic effects of TPO in stimulating the development and maturation of megakaryocytes, resulting in increased platelet count. It is approved by the European Medicines Agency (EMA) and the US Food & Drug Administration (FDA) for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure, and for the treatment of thrombocytopenia in adult patients with chronic immune thrombocytopenia (ITP) who have had an insufficient response to a previous treatment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal